The Heart of Medical Innovation
Medical progress is often described in terms of great discoveries and game-changing treatments. Yet, at the center of every breakthrough are people who choose to participate in clinical trials. Volunteering for research isn’t just about passively receiving a new drug or therapy; it’s an active commitment to advancing knowledge and shaping the future of medicine. From developing life-saving vaccines to fine-tuning cancer treatments, every innovation began as an idea tested through rigorous study.
For those considering their role in research, an initial question might be: should I participate in clinical research? This decision is personal and multi-faceted. Some join to access novel therapies before they become widely available. In contrast, others are driven by the resolve to help future generations or make a difference for people with similar health challenges. Clinical trials continue to be a vital resource for scientific progress and anyone hoping to play a direct part in transforming healthcare.
Bridging Gaps in Healthcare
Today’s healthcare system isn’t without its challenges or unanswered questions. New illnesses emerge, chronic conditions persist and sometimes, existing treatments do not address specific populations or unique symptoms. Clinical trials are the crucial link between cutting-edge science and everyday patients, filling in gaps that other research cannot. They’re uniquely designed to test medical innovations in real-world environments, moving discoveries from the test tube to patient care.
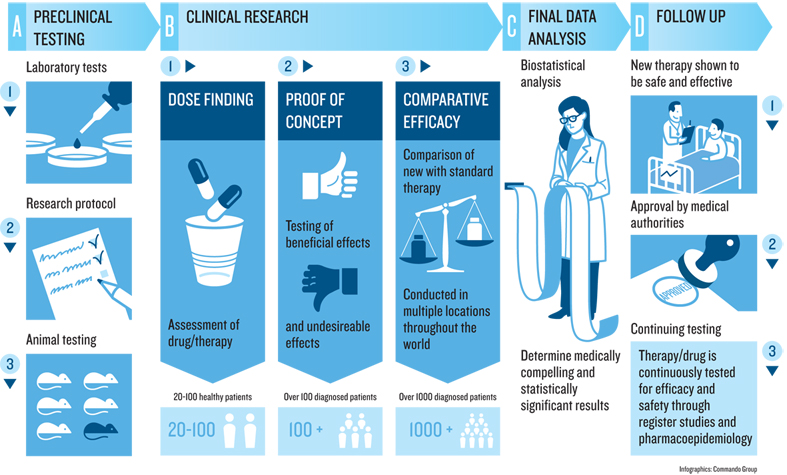
The National Institutes of Health (NIH) stresses the importance of these studies by detailing how each phase in the research process—starting with early laboratory work and ending in human studies—helps ensure new treatments are safe and effective. Thanks to the willingness of trial volunteers, innovative therapies for rare illnesses, improved medications for common diseases, and solutions for underserved communities have become possible. Without participant involvement, this essential jump from the laboratory to the bedside would stall, and healthcare improvements would remain out of reach.
Becoming a Volunteer
- Who can volunteer? Clinical research welcomes people from all walks of life. Trials need diverse contributors: healthy adults, children, seniors, and people living with various medical conditions.
- What’s involved? Before joining, every volunteer undergoes an in-depth screening process and receives detailed information. The research team reviews the study’s purpose, length, procedures, and possible risks or side effects to ensure everyone makes an informed choice.
- Why participate? Motivations vary. Some seek access to groundbreaking therapies, while others want to support family members or pay it forward. In many cases, participation brings a unique sense of contribution and community.
For instance, participants in diabetes research have helped create safer insulin formulations, while volunteers in rare disease studies can spark hope where little previously existed. Each person’s involvement, big or small, truly matters, and their contributions ripple outward, improving health for countless individuals beyond the trial itself.
The Value of Diverse Participation
Medical discoveries can only benefit the broader population if clinical trials reflect the diversity found in real-world communities. Historically, many groups—such as people of color, women, and older adults—have been underrepresented in research studies. This underrepresentation limits the accuracy and equity of new treatments, potentially leading to therapies that do not work equally well for everyone. The urgency for truly diverse participation highlights that inclusive research leads to better results. Differences in genetics, lifestyles, and social circumstances can affect how people respond to treatment, making it vital to include varied perspectives in every study phase. When trials embrace individuals of all backgrounds, their results become more relevant, robust, and transformative, ensuring no group is left behind as science moves forward.
Personal Benefits and Considerations
Volunteering for a clinical trial often offers more than meets the eye. For some, it means receiving comprehensive health assessments and extra medical supervision from top research teams—insights and attention that can improve overall well-being. In cases involving serious illness or rare conditions, early access to promising treatments can present new hope and, in rare instances, deliver real medical improvements sooner than waiting for general market release.
- Potential to access cutting-edge therapies not yet approved elsewhere
- Personal monitoring by experienced clinical investigators
- Opportunity to learn about your health status through advanced testing
- Pride in helping bring about change for others in similar situations
These potential benefits come with essential considerations. Research studies are rigorous because new treatments may have side effects or prove ineffective in specific participants. Open communication with research teams and personal healthcare providers is crucial. Reading all documentation and asking honest questions helps make informed decisions, minimizing surprises and promoting a positive experience for everyone involved.
Maintaining Safety and Ethics
Robust standards guide today’s clinical trials to protect every volunteer. Strict ethical codes and regulatory oversight ensure that participants receive clear, honest information and undergo careful monitoring at all stages. This marks a dramatic transformation from earlier eras, adding extra layers of support and safety that empower more people to participate without fear.
- Informed Consent: Each participant learns about study details, risks, and benefits, and is reminded of their right to leave at any point, for any reason.
- Oversight Committees: Panels of independent experts review all trial materials and practices to guard participant safety and research integrity.
- Ongoing Health Checks: Researchers actively monitor every volunteer’s health, responding quickly to any issues.
- Respect for Autonomy: The participant always has complete control over their involvement and may withdraw consent at any time with no repercussions.
These safety nets build trust and reinforce the value placed on each person’s well-being. Volunteering in a clinical trial is meaningful and safer than at any point in research history.
Moving Forward in the Clinical Trial Landscape
Technology is transforming the clinical trial landscape, opening doors to new participation methods and making studies more accessible. Telemedicine, wearable health devices, and remote data monitoring allow individuals who may have struggled with transportation or time constraints to participate from the comfort of their own homes easily.
With greater transparency and communication, public interest rises, improving participation rates and study quality. Each forward step in clinical trials, primarily as technology lowers barriers, signals a more inclusive future—one where medical progress represents and benefits all people, regardless of background or location.